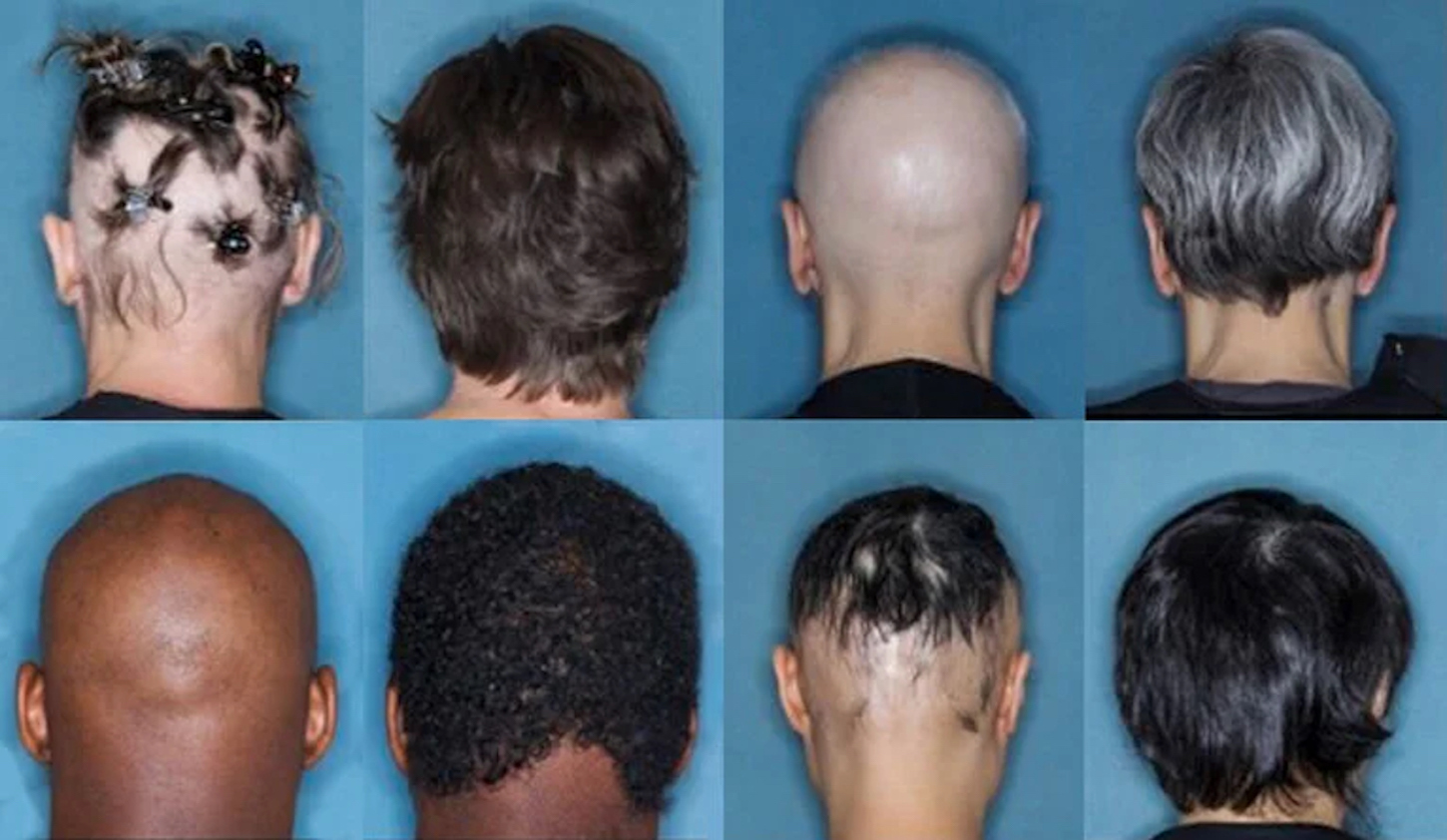
In June 2014, the publication of a new paper stirred enormous excitement around the world. Using tofacitinib citrate, an FDA-approved drug for rheumatoid arthritis, Brett King, MD, PhD, associate professor of dermatology, gave Kyle Rhodes, a 25 year old struggling with severe alopecia areata, his hair back. Once bare from head to toe, the young man finally had a full head of hair, eyebrows, and eyelashes, as well as facial and armpit hair. Other patients in Rhodes’ shoes, who until then had essentially zero options, rejoiced that for the first time, their condition might be treatable. Patients from all over the US and the world reached out to King seeking help, and only weeks later they were traveling to see him. He wrote prescription after prescription after prescription.
Every single one was denied by insurance.
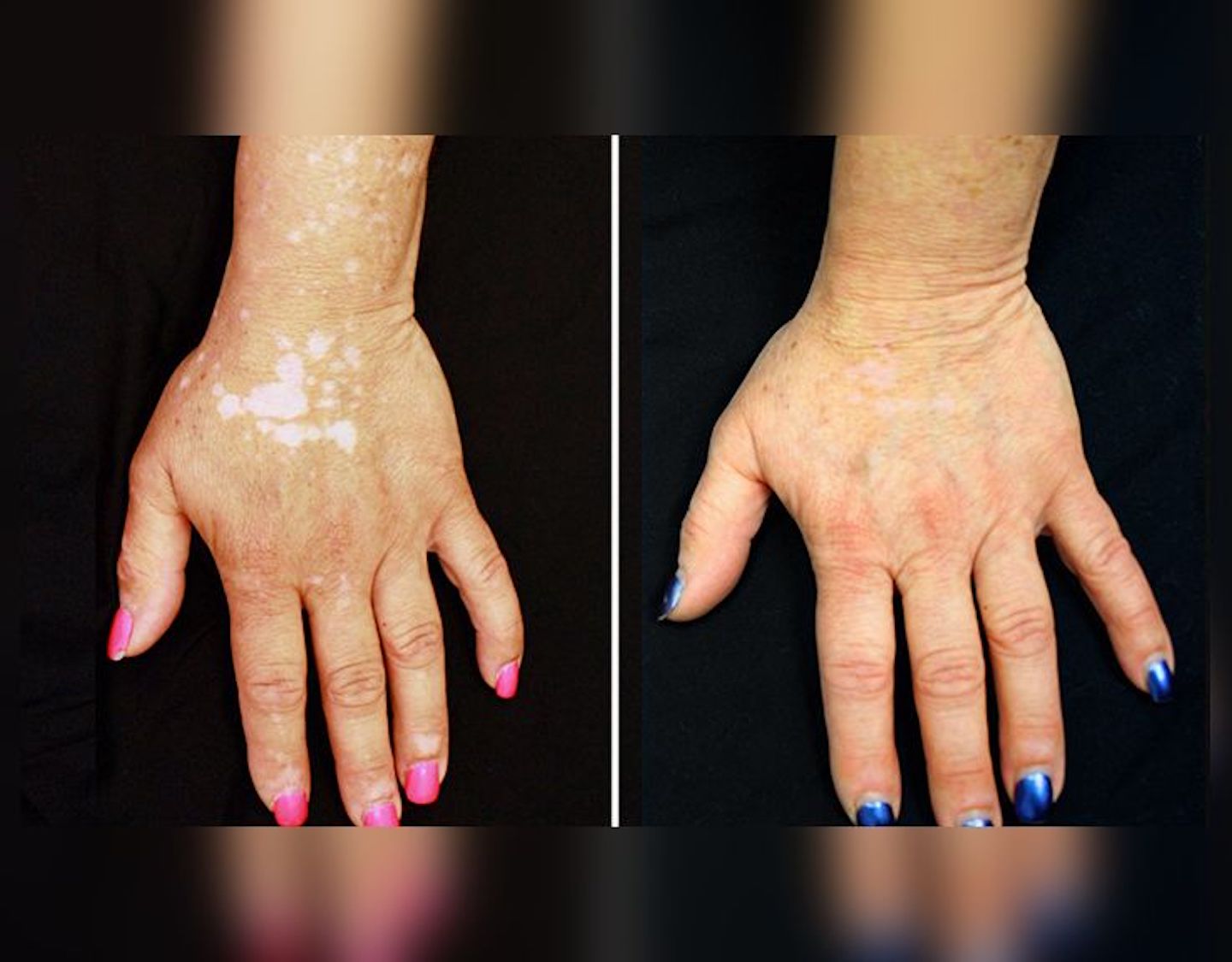
A year later, King published a report showing successful treatment of a patient with severe vitiligo, again with tofacitinib. Again, patients came for treatment and, again, every single one was denied by insurance.
Skin diseases such as alopecia areata and vitiligo, a condition in which there is patchy loss of pigment in the skin (leading to white spots), can be disfiguring and severely impact life for those who suffer from them (and their family members). And treatments were lacking, so when King made the discovery in 2014 that a class of drugs known as Janus kinase (JAK) inhibitors, which at the time was used to treat rheumatoid arthritis, could dramatically restore normal appearance to patients, the scientific and medical communities took notice.
A new challenge emerged for King—convincing health insurers to cover these treatments for diseases they were not intended for. He spent countless hours at his desk drafting thousands of appeal letters to insurance companies and advocating for his patients. But convincing them was not easy.
Alopecia and Vitiligo Are Life-Altering
Alopecia areata is an autoimmune disorder that typically manifests as round patches of hair loss. In severe cases, it can lead to complete loss of all hair on the body, a condition sometimes called alopecia universalis. But even mild forms of alopecia can significantly impact patients. “Imagine a nickel-sized spot of hair loss,” says King. “If it’s at the top of somebody’s head, it may never be noticed. But if you put that same spot in the middle of someone’s eyebrow, all of a sudden, that person is getting a lot of unwanted stares and attention.”
When that nickel-sized spot turns into 10 or 20 spots, or into complete hair loss, the condition becomes disfiguring, King says. People equate severe hair loss with illness, even when most everyone lacking hair is not ill. “If you go to a children’s cancer hospital website, what do you see? You see hairless children,” he says. “We all recognize the hairless person as being somebody who is sick because somewhere deep down inside, we feel that humans should have eyebrows framing their face and hair on their head.” Considering these things, it is not surprising that people struggling with alopecia frequently also experience depression and anxiety.
Like with alopecia, vitiligo affecting some body parts is relatively hidden, but when white spots are on the face or hands, it can be devastating. Michael Jackson’s struggle with vitiligo is one of the best-known cases. The singer faced heavy scrutiny from the public about his appearance and many speculated the change was due to his wanting to look white. “He didn’t want to be white. He just didn’t want to have white spots,” says King. “This is an amazing example of how you can be one of the world’s greatest entertainers, amazingly talented and wildly rich and famous, but it is intolerable to look different.”
Treatment of alopecia areata and vitiligo was “in the Dark Ages,” says King. There were no medications available that were specific to the underlying disease process. And as such they were often, if not usually, ineffective.
Identifying a Revolutionary Treatment
King met Kyle Rhodes in 2013, when Rhodes was referred to King for psoriasis treatment. He also suffered from severe alopecia areata. King knew that tofacitinib, a JAK inhibitor approved just a year earlier for rheumatoid arthritis, was showing success in clinical trials for treatment of psoriasis, which is another autoimmune disease. Research was showing that the drug was effective in reversing alopecia areata in mice as well.
Less than a year after he started treatment, Rhodes’ hair was fully restored. King believes JAK inhibitors work to treat alopecia by blocking the immune system from attacking hair follicles.
Then, a year later, King successfully treated 53 year old Linda Lachance, who suffered from vitiligo, with the same drug. She suffered with white spots all over her face, hands, and body that were growing in number. Within five months after she sought treatment, nearly all of the spots on her face, arms and hands disappeared. “The before and after pictures in both of these cases were so dramatic,” says King. “We showed hair where there was none, we showed color where there was only white. They were so awe-inspiring that they captured the world’s attention. You didn’t have to be a dermatologist to appreciate the difference.”
Advocating for Insurance Coverage for Alopecia and Vitiligo
Insurance companies, however, were less impressed. Alopecia and vitiligo are cosmetic conditions, they argued. And furthermore, the drug was approved for rheumatoid arthritis, not these skin conditions. They denied coverage for all of his patients. “On the other side of that denial, there is a life that’s being affected,” says King. “It’s horrible to watch suffering, depression, and anxiety and to see a path to make it better, but you can’t cross the bridge to do it.”
It became harder and harder to argue with the evidence. As time went on, the momentum behind this grew and grew.
Brett King, MD, PhD
In appeal letters written to insurance companies, King detailed the life-altering effects of the diseases and the evidence supporting JAK inhibitors as treatment. His first vitiligo publication was covered by the BBC, where a physician said “single case reports like this are sort of seen as the lowest of the low, really, in terms of evidence.” King understood this but believed these cases were more than chance observations. “In each instance there was real science to support the observation.”
The before and after photos did, however, capture the attention of the media. King appeared on TV programs ranging from Fox and Friends in New York City to the BBC. “Early on, I think I was truly alone in this,” King reflects. “I’d tell audiences of my peers ‘I’m about to tell you about a drug that doesn’t exist in dermatology for a disease that you may not care much about. And I hope to convince you why you should work really hard to get this drug for your patients even though it is almost impossible to get.’”
Through sheer persistence, a small number of King’s appeal letters began getting approved. Then, in 2016, he published a study on 90 patients with alopecia areata that demonstrated tofacitinib’s success in treating the condition. This piqued the interest of many people, and even drug companies such as Pfizer and Eli Lilly were taking notice. “It became harder and harder to argue with the evidence,” says King. “As time went on, the momentum behind this grew and grew.” says King.
King went from having all his patients being denied coverage to half of them getting their treatment approved. “I wrote to the insurance companies that denied treatment for my patients and said ‘No, no, no, no,’” he says. “And over time I heard back ‘Yes, yes, yes.’”
Covering New Treatments Isn’t as Simple as It Might Sound
While it may seem easy to point fingers at insurance companies, the burden of high health care costs is an extremely complicated issue with many players involved, says Howard Forman, MD, professor of radiology & biomedical imaging and in the Institute for Social and Policy Studies. “New innovation is miraculous. It can change lives. But ultimately, someone has to pay for it,” Forman says. “Insurance companies have their own priority to hold down costs for the beneficiaries. In an ideal world, pharmaceutical companies and insurance companies would partner together to make treatments more affordable for those who can’t otherwise afford it, but there are no simple solutions.”
Doctors, he says, can help move that process forward. “Physicians who have the greatest experience with these patient populations should do everything they can to advocate to those who can help those populations,” says Forman. “That includes to the pharmaceutical companies to provide compassionate provision of expensive drugs and to the insurance companies to work on their behalf to be able to provide for the maximum number of people who can possibly benefit in an affordable way.”
FDA Approvals Are Victories for Patients
On June 13, 2022, eight years after the publication of King’s initial paper, the FDA approved the JAK inhibitor baricitinib for treatment of alopecia areata. On July 22, 2022, it approved ruxolitinib cream for vitiligo. With those approvals, King says, approximately 80% of his patients now receive insurance coverage for their treatments.
Now that effective treatments are finally available to patients, the next step, says King, is raising awareness in the health care community. “Sometimes, when diseases have a long history of being untreatable, we diminish them,” he says. “That’s what we did with alopecia areata and vitiligo. We said they were cosmetic. So now part of the challenge ahead is educating the medical community that these are real diseases, and they deserve our care and attention. And they deserve treatment as much as other things we don’t hesitate to treat, like psoriasis or eczema.”
And the search for treatments hasn’t ended. While overall, JAK inhibitors have been an enormous success, they don’t work for all patients. King wants effective treatment for these patients as well. “We’re in a historic place and we should be celebrating that,” he says. “But we need to continue to advance discovery so that everyone can be treated.”
Meanwhile, King says, self-advocacy among patients will be especially important. “Five years from now, there will be a whole generation of doctors who will have trained using these therapies and those doctors will be comfortable using them,” he says. “In a decade these medicines will be commonly used. But for now, it’s more important than ever for patients with these conditions to educate themselves and to find dermatologists who are knowledgeable about these medicines.”