











About EQBMED
Equitable Breakthroughs in Medicine Development (EQBMED) is a collaborative partnership committed to making medical research more accessible. We are working so those who wish to participate in clinical trials have the opportunities to do so.

The Mission
EQBMED’s mission is to engage communities that have historically been less involved in the research and development of potentially life-saving medical treatments, diagnostics, and more.
Our goal is to create a replicable framework of community-based clinical care sites equipped with tools and resources for sustainable participation in clinical research. This effort aims to reshape the landscape of clinical trials and medical research, bringing research opportunities closer to underserved communities and ensuring their active participation in the development of medical advancements. We define underserved communities as those with a disproportionate burden of disease and barriers to accessing high-quality health care and research. Individuals from underserved communities may include those from minority groups, those who have a low income, or those who have limited access to healthcare and research based on geography, insurance coverage, physician supply, limited health literacy, and other factors.
To achieve this, EQBMED partners with experts including scientists, physicians, community and professional society leaders, FDA leaders, and industry executives who are leading thinkers on clinical research, clinical trial operations and access, community-based care models, and more. These partners work together to expand clinical trial access, generate valuable insights, and share learnings with the broader clinical trials community.
The Network Partners
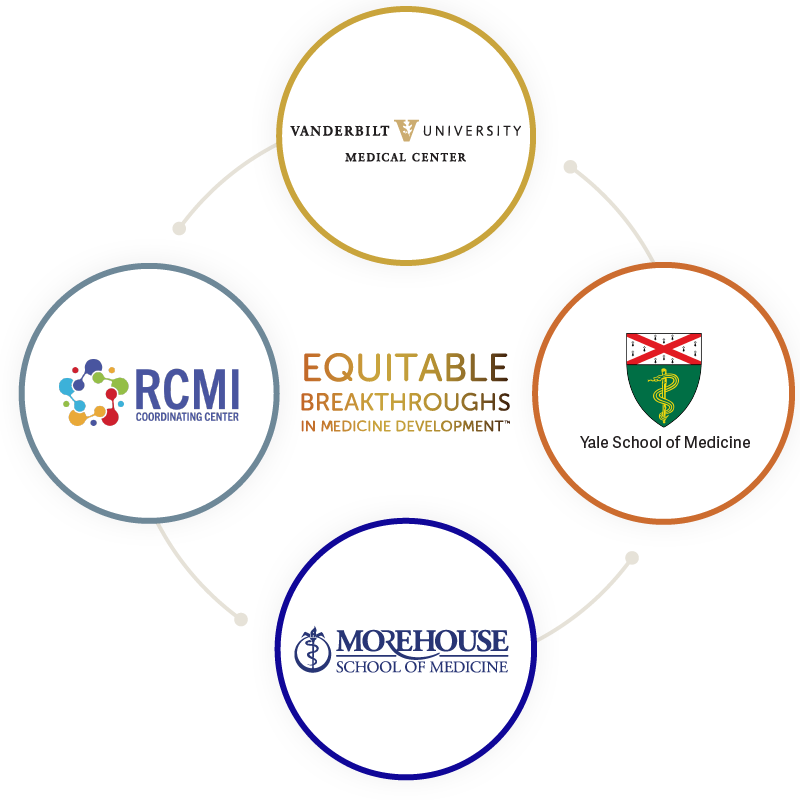
EQBMED is led by the Yale School of Medicine, Morehouse School of Medicine, the Research Centers in Minority Institutions Coordinating Center (RCMI-CC) at Morehouse School of Medicine, and Vanderbilt University Medical Center, referred to as Network Partners, who are coordinating the initiative.
EQBMED uses a multi-stakeholder model to take a holistic approach, working with the Network Partners to support local site readiness and sustainability.
Key elements for making local sites sustainable in the clinical trial ecosystem include:
- Building on existing strengths and capabilities using a co-developed maturity model
- Developing lasting partnerships
- Providing continuous mentoring support from EQBMED Network Partners to help sites meet their goals and aspirations
Pharmaceutical Research and Manufacturers of America (PhRMA) awarded a $10 million grant to Yale University to support the creation and development of this initiative. PhRMA convened thousands of stakeholders in the process to design this learning phase to work with 10 sites in underserved communities to help enhance diverse participation in clinical trials and uncover learnings to promote sustainability and success.




