Yale scientists have identified a crucial biological link in the process that transforms certain complex sugars into hematopoietic stem and progenitor cells.
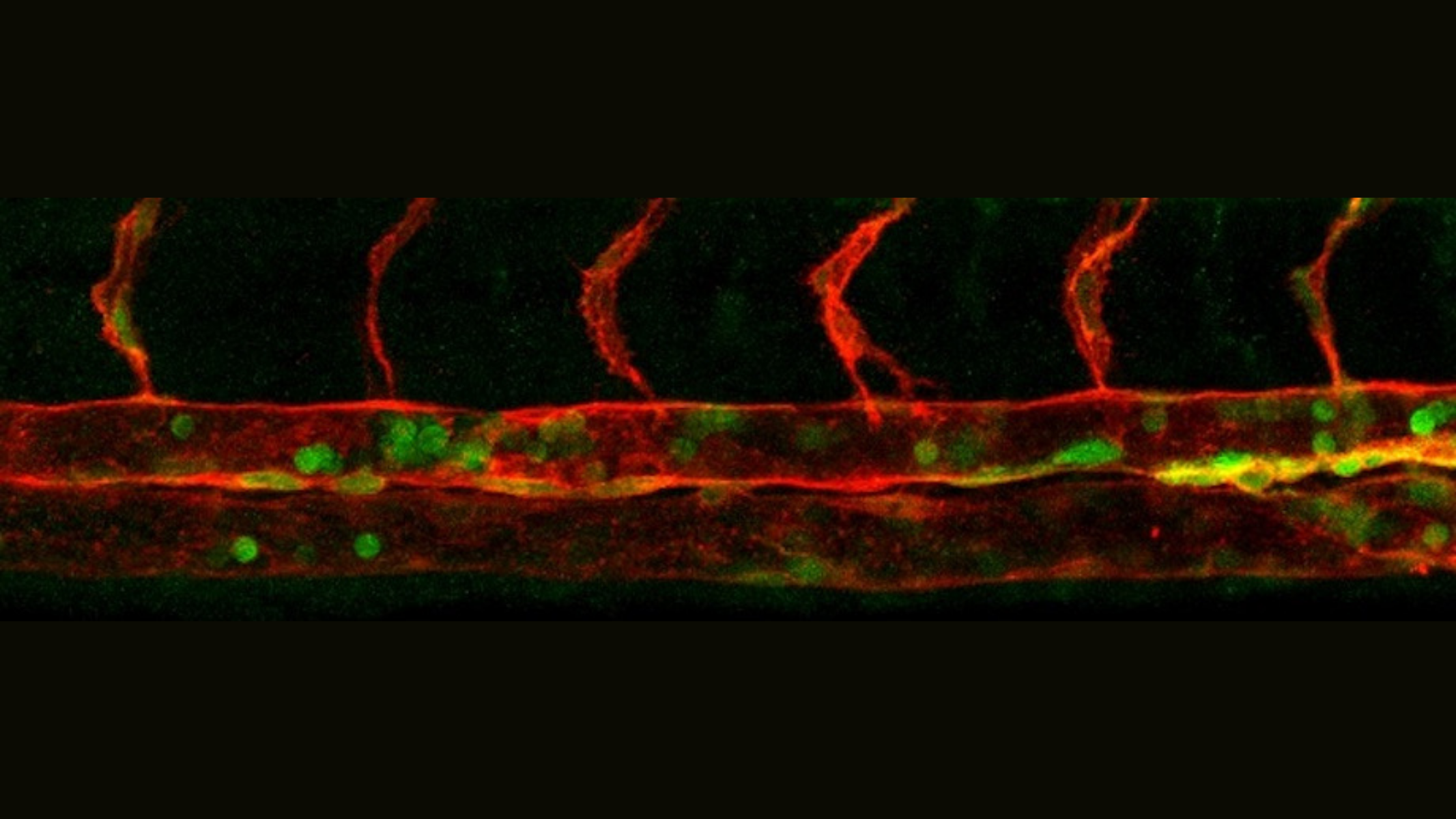
Reprogramming cells into hematopoietic stem and progenitor cells (HSPCs) has been the holy grail for autologous stem cells transplants, a lifesaving therapy for blood cancer diseases. However, the mechanisms that regulate this transition at the cellular and molecular level are poorly understood. A new study published in Science identifies microRNA (miR)-223 as a crucial link between complex sugars called glycans and endothelial-to-hematopoietic transition (EHT), a process where hemogenic endothelium differentiates into hematopoietic stem and progenitor cells (HSPCs).
Advances in the stem cell field
A breakthrough in stem cell transplantation occurred in the 1960s when biologist Ernest McCulloch, MD and biophysicist James Till, PhD discovered that hematopoietic stem cells (HSCs) have the ability to self-renew and eventually produce different types of blood cells. These stem cells can form all blood cell lineages needed throughout life.
A second milestone was the discovery of biomolecules made from sugar. Specific cell surface glycans play an important role in human biology from how our body recognizes and fights disease to cell cycle progression.

Deciphering the sugar code
By combining analytical, structural, and cell biology techniques, the researchers found that proteins on the surface of endothelial cells are modified by a specific “sugar code” called N-glycan to limit their blood production. This common biomolecule limits the activity of hematopoietic regulators.
There are two potential applications for this discovery. First, it provides an improved method for the production of stem cells with a higher chance for success. Second, it could have a wide application for cancer treatments, allowing scientists to use glycoengineering to change the surface of the cell and see how it affects the progression of cancer.
“We can use genetics or chemicals to alter this “sugar code” and change the efficiency of blood production in embryos. These findings will inform strategies to understand the production of blood stem cells in the lab, which can be used to treat blood diseases, such as leukemia,” Nicoli said.
The results require further analysis to identify treatment targets for blood disorders. “This study is a step towards better understanding the signaling pathways that regulate HSPC production and differentiation, which will be critically important to inform early interventions and therapeutic agents,” said Dionna M. Kasper, PhD, postdoctoral fellow at the Nicoli lab and first author on the study.
These findings will inform strategies to understand the production of blood stem cells in the lab, which can be used to treat blood diseases, such as leukemia.
Stefania Nicoli, PhD
This study was conducted in collaboration with the Department of Genetics, the Yale School of Medicine Vascular Biology & Therapeutics Program, and Karen Hirschi, PhD, professor adjunct of cardiovascular medicine.