Yibing Qyang, PhD, has received $2 million in medical research funding from the Department of Defense. The project proposes to use human induced pluripotent stem cells (hiPSCs)-derived heart muscle cells to improve the pumping activity of tissue-engineered pulsatile conduits.
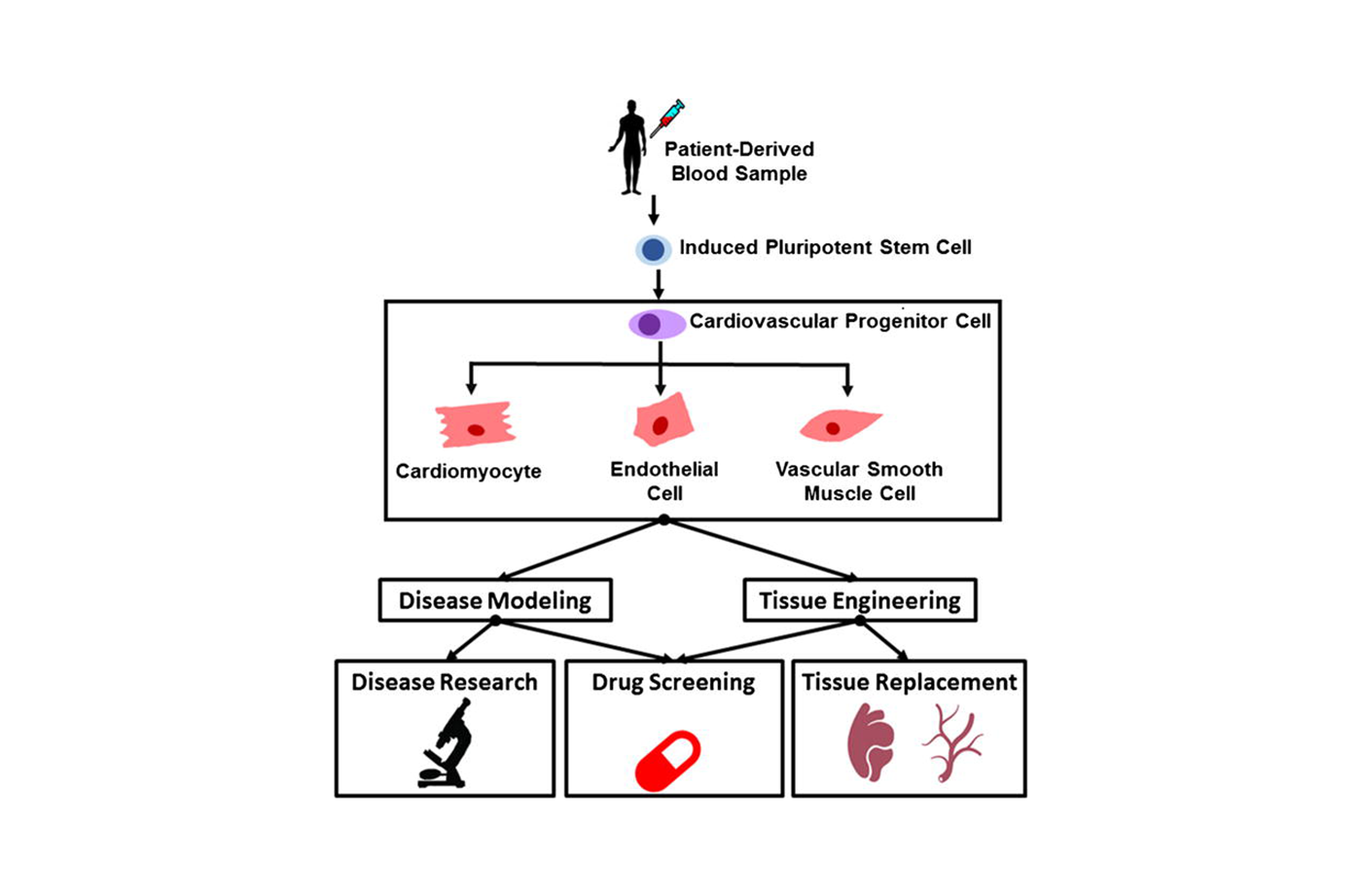
The development of hiPSCs was a turning point in stem cell research and tissue engineering. Pluripotent stem cells are capable of “differentiating” or transforming into other types of cells. In 2006 Shinya Yamanaka, MD, PhD, succeeded in reprogramming skin cells into pluripotent stem cells that could be coaxed into cells of nearly any human tissue. This feat was awarded the Nobel Prize in 2012. With this finding, stem cell scientists and engineers are developing replacement tissues that are a genetic match for each individual patient.
The same year as Yamanaka’s breakthrough, Yibing Qyang, PhD, was a researcher at the Harvard Stem Cell Institute. Qyang studied the cellular and molecular mechanisms that contribute to cardiovascular disease. Now an associate professor of medicine (cardiology) and pathology and a faculty member at the Yale Stem Cell Center, Qyang’s group is engineering a stem cell-based device to help children with life-threatening cardiovascular diseases.
Each year, more than 35,000 children in the U.S. are born with congenital heart disease (CHD). Three decades ago that diagnosis was fatal. Today 300 million people live with CHDs, through corrective surgery, but still suffer from symptoms related to decreased heart output.
Single-ventricle congenital heart disease (SVCHD) is a common heart-related birth defect. The patient is born with one functional ventricle. Single ventricle anomalies cause oxygen rich and poor blood to mix, which prevents tissues from receiving enough oxygen.
If untreated, the stress on the ventricle often leads to heart failure. The condition affects 1 in 1,000 births, and this population is growing. “Human iPSC provides a unique resource for generating patient-specific cardiomyocytes [cardiac muscle cells] to study cardiac disease mechanisms and provide surgeons autologous engineered heart tissue for treatments,” according to Qyang.
A Model for Single Ventricle Congenital Heart Defects
Children born with this condition have a 70 % chance of premature death without surgery. A cardiac surgeon performs the Fontan procedure, a series of three operations that can treat the condition. A tissue-engineered vascular conduit creates the connection to allow deoxygenated blood to directly enter the lungs. Although the operation improves survival rates, engineered vascular grafts cannot pump blood, which results in insufficient blood flow to tissues, heart failure, and pulmonary vascular disease. To generate a mechanism that creates enough pressure to pump blood into the lungs, Qyang collaborated with Stuart Campbell, PhD, associate professor of biomedical engineering and cellular and molecular physiology; Laura Niklason, MD, PhD, the Nicholas Greene Professor of Anesthesiology and Biomedical Engineering; Albert Sinusas, MD, professor of medicine (cardiology), radiology and biomedical imaging; George Tellides, MD, PhD, professor of surgery and investigative medicine; and Robert Elder, MD, associate professor of pediatrics and medicine and director of the Adult Congenital Heart Program. With hiPSCs, scientists are able to collect blood cells from the patient and develop tissue-engineered grafts that are an exact genetic match for each individual. This means that the tissue can be implanted without fear of rejection, as seen in donor organs.
The project builds off of a $300,000 medical research seed grant awarded by the Department of Defense (DOD) in 2015. Qyang and his team produced tissue-engineered pulsatile conduits (TEPCs) by wrapping contracting engineered heart tissue (EHT) around a cell-free vascular scaffold, followed by electrical and mechanical training and maturation in a bioreactor. TEPCs use fibroblasts removed from the patient’s skin or blood cells to engineer patient-specific induced pluripotent stem cells. As surgeons only need the engineered tissue constructs two to three years after the initial surgery (graphic below), the Qyang group has a feasible time window to develop an autologous iPSC-based, mechanically active Fontan conduit tailored to the individual. This device may provide a curative therapeutic intervention for SVCHD patients, thus lowering the lifetime cost of care. Preliminary results showed these TEPCs are capable of increasing the pressure, which has a direct impact on the rate of blood flow.
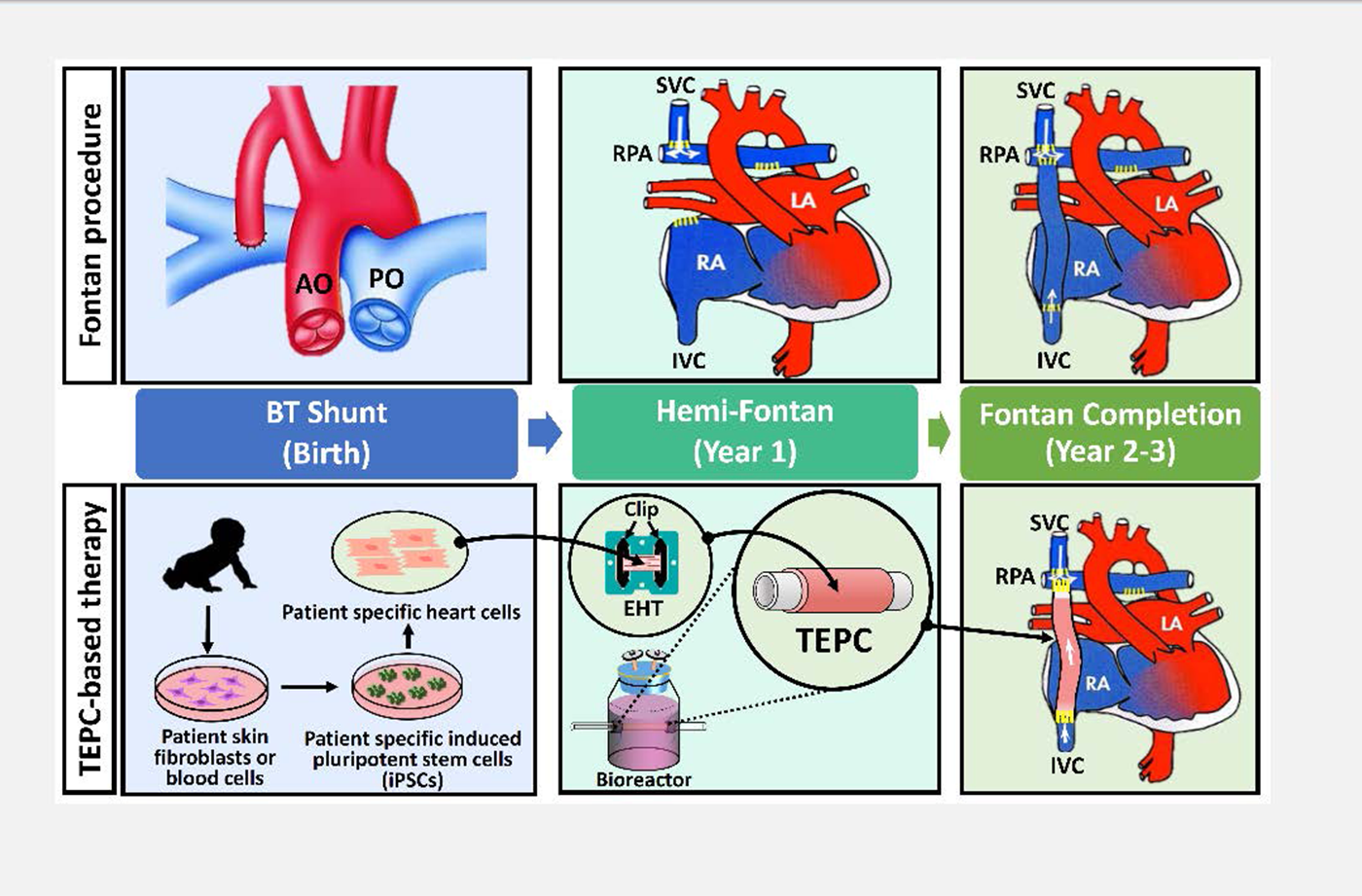
Figure 1 Fontan procedure: Babies with single ventricle heart defects are often treated with the Fontan procedure. Surgeons perform a series of open heart procedures to re-route blood flow from the superior and inferior vena cava directly into the pulmonary artery.
- Stage 1 (Birth) The Blalock–Taussig Shunt procedure is temporarily used to modify blood pressure into the lungs.
- Stage 2 (Year 1) During the Hemi-Fontan operation, a surgeon connects the superior vena cava (SVC) directly to the pulmonary artery.
- Stage 3 (Year 2-3) In the final phase, a synthetic graft is used to make the connection between the inferior vena cava (IVC) and the pulmonary artery. A small hole, or fenestration, is generally created between the vascular conduit and the right atrium. This allows the increased pressure of the atrium to help drive deoxygenated blood into the lungs without passing through the heart.
Figure 2 TEPC-based therapy: Illustration of cell-based therapy for cardiac disease. Infants born with single ventricle congenital heart defects have a weakened pumping ability. The Qyang lab uses patient-specific heart muscle cells to build a beating conduit for the Fontan surgery. They intend to use a bioreactor that trains heart muscle cells to pump blood for the patient.
- Stage 1 (Birth) Blood samples from the patient are used to generate Human iPSCs.
Human iPSC provides a unique resource for generating patient-specific cardiomyocytes [cardiac muscle cells] to study cardiac disease mechanisms and provide surgeons autologous engineered heart tissue for treatments.
Yibing Qyang, PhD
- Stage 2 (Year 1) In the second phase, scientists produce tissue-engineered pulsatile conduits (TEPCs) by wrapping engineered heart tissue (EHT) around a cell free vascular scaffold. TEPCs are trained in a bioreactor system that provides electrical and mechanical stimuli to mimic the natural environment heart muscle cells develop in.
- Stage 3 (Year 2-3) The trained tissue is implanted into the patient. This biological strategy can improve the tissue’s ability to drive blood flow into the lungs.
Repairing the Human Heart
Qyang joined Yale in 2008, as a principal investigator with Cardiovascular Medicine and the Yale Stem Cell Center. A year later, Yale became the first Food and Drug Administration-approved site to implant synthetic blood vessels grown from a patient’s bone marrow cells back into the patient. Qyang also organizes the Yale Stem Cell Research Forum, a monthly seminar that provides a platform for Yale scientists to discuss research activities. Qyang’s laboratory also created a replica blood vessel, hiPSC-derived smooth muscle cell-based tissue rings that could be used to test the effectiveness of potential cardiovascular therapies. This vascular ring allows a physician to observe how a patient will respond to approved FDA drugs before prescribing it. The smooth muscle cell research is a long-standing collaboration with Laura Niklason, MD, PhD, in the Department of Anesthesiology.
Qyang has received $2 million in peer-reviewed medical research funding from the DOD in 2019. The current project proposes to use hiPSC-derived heart muscle cells to improve the pumping activity of tissue-engineered pulsatile conduits. First, a bioreactor system stimulates the heart tissue. This sends a signal to the heart cells that mimic the natural environment in which heart muscle cells develop prior to birth. Over time, this strategy is expected to improve the force output of the TEPCs to improve blood flow into the lungs.
For initial testing, TEPCs initially will be implanted into the venous circulation of rats with a defective immune system to avoid rejection. These experiments test the feasibility of these tissues to improve blood circulation in a living system. The pilot study will generate important data for the future clinical application of hiPSC-derived TEPCs. Other collaborators on this project include Jinkyu Park, a postdoctoral researcher at Yale School of Medicine, Chris Anderson, a graduate student in Yale’s Integrated Graduate Program in Physical and Engineering Biology, Lorenzo Sewanan, an MD/PhD graduate student at Yale, Mehmet Kural and Yan Huang, associate research scientists at Yale Cardiovascular Medicine, and Jiesi Luo, an American Heart Association postdoctoral fellow at Yale.